One of the most promising emerging therapeutics are stem cell derived extracellular vesicles (EVs)1. EVs are are produced by virtually all cell types and contain heterogeneous populations of both plasma membrane-shed microvesicles (50-1000 nm in size) and exosomes (40-150 nm in size) which are derived from the endocytic pathway. EV cargoes including bioactive lipids, microRNAs, and transmembrane proteins make EVs a potent neuroprotective and regenerative therapy 2,3.
In terms of treating neural injury specifically, the vast majority of studies have evaluated the therapeutic efficacy of EVs derived from mesenchymal stem (MSC) sources5-6. However, in vivo biodistribution of EVs is highly dependent on cell source, thus suggesting EVs will display specific biodistribution patterns that reflect their parent cell line7. Consequently, the TNRR Laboratory decided to compare the neuroprotective and regenerative properties of neural stem cell (NSC)-derived EVs versus MSC EVs post-stroke in rodents8. We found MSC EV treatments trended toward decreasing stroke lesion volume, whereas NSC EVs significantly decreased lesion size, preserved motor function, and improved episodic memory. These findings collectively warranted further rigorous testing of NSC EVs in our pig ischemic stroke model.
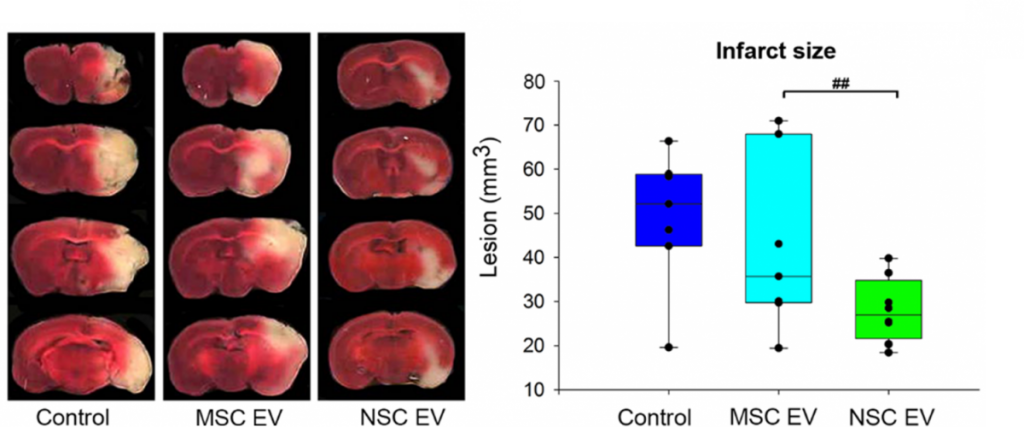
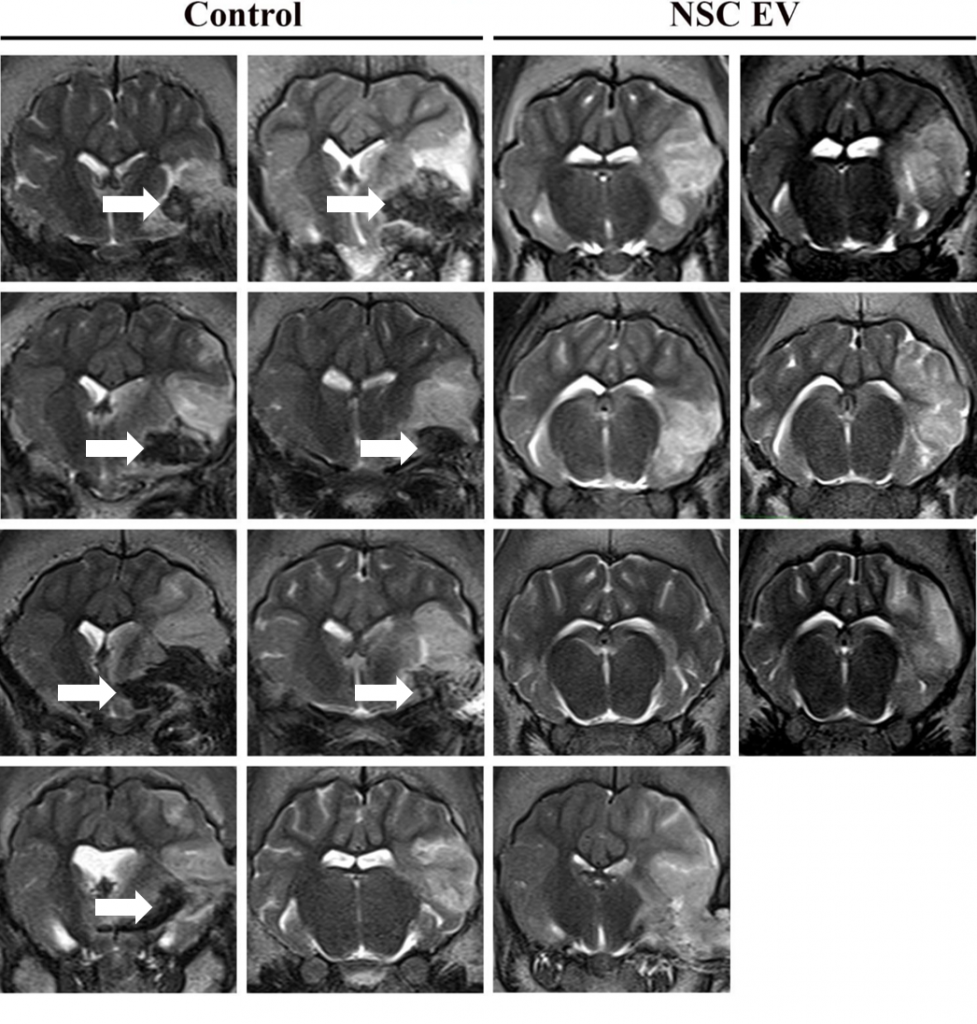
In this follow-on pig study9, the TNRR Laboratory demonstrated NSC EVs were a potent biological treatment that positively impacted both molecular and functional post-stroke outcomes. NSC EVs in our porcine ischemic stroke model exhibited a multifactorial effect leading to decreased lesion volume, hemispheric swelling, and intracerebral hemorrhage while also promoting diffusivity, white matter integrity, and functional recovery in a large animal model with similar cerebral architecture and white matter composition to stroke patients. As an effective treatment in both rodent and porcine stroke models, the TNRR Laboratory provided unprecedented evidence that NSC EVs possess inherent biological characteristics suitable for translation into human stroke therapeutics.
- 1Lener T., et al., Applying extracellular vesicles based therapeutics in clinical trials-an ISEV position paper. J Extracell Vesicles, 2015. 4: p. 30087.
- 2Basso M., et. al., Extracellular vesicles and a novel form of communication in the brain. Front Neurosci, 2016. 10: p. 127.
- 3Raposo G., et.al., Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol, 2013. 200: p. 373–383.
- 4Doeppner T., et. al., Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med, 2015. 4: p. 1131–1143.
- 5Xin H., et. al., Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab, 2013. 33: p. 1711–1715.
- 6Zhang Y., et. al., Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int, 2017. p. 111:69–81.
- 7Wiklander O., et al., Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles, 2015. 4: p. 26316.
- 8Webb R., Kaiser E., et. al., Human neural stem cell extracellular vesicles improve tissue and functional recovery in the murine thromboembolic stroke model. Transl Stroke Res, 2018. 9(5): p. 530-539.
- 9Webb R., Kaiser, E., et. al., Human Neural Stem Cell Extracellular Vesicles Improve Recovery in a Porcine Model of Ischemic Stroke. Stroke, 2018. 49(5): p. 1248–1256.