In addition to the Translational Neural Repair and Regeneration Laboratory’s ischemic stroke, traumatic brain injury, and alcoholism pig models, the anatomical similarities between pigs and humans also allows for the study of alternative human pathophysiological processes. The Translational Neural Repair and Regeneration Laboratory is available to assist and provide guidance for our clients’ research endeavors even if they fall outside the field of neural repair and regeneration.
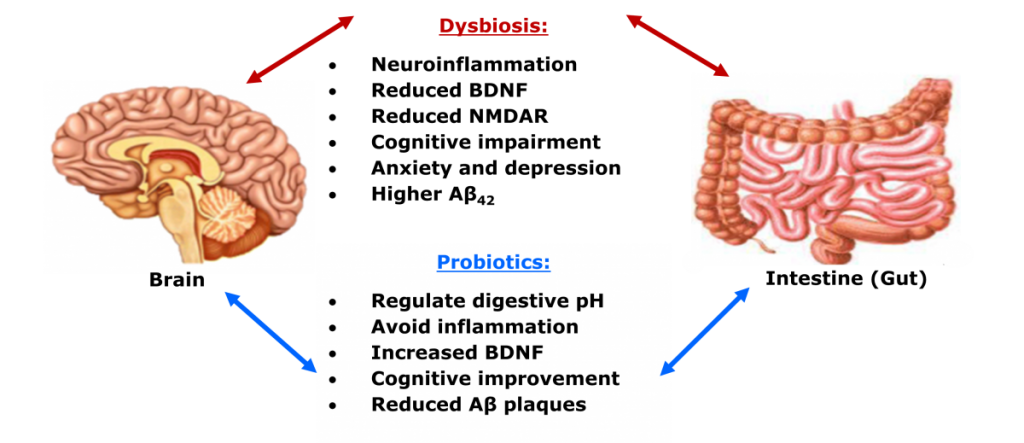
Gut microbiome alterations in response to neural injury have recently been associated with disease pathology and susceptibility. However, changes in the microbial community in response to neurological diseases have not been well characterized. The research conducted in our laboratory aims to define the protective role of bioactive food compounds and their mechanisms with the underlying goal of translating experimental findings into practical recommendations that promote optimal health in humans. The following have been investigated.
- Assessment of dynamic changes in the gut microbiome composition and diversity at the acute stage of ischemic stroke in a pig model
- Bioavailability and pharmacokinetic studies to test green tea catechins with various dietary fat contents in a pig model
- Phytochemicals were tested as a potential therapeutic agent in a pig ischemic stroke model
- Cognitive development and gut-brain interaction following maternal intake of bioactive food compounds during gestation and lactation in pigs
Please refer to the Bioactive Food Compounds and Health Laboratory for more information.
Musculoskeletal healing and enhancement in large animals models has played an integral role in advancing our current understanding of the musculoskeletal system and associated biomechanics. The porcine model in particular has been increasingly utilized for regenerative medicine and tissue engineering of specific joints, including the knee and temporomandibular joints, as well as tissues, such as bone, cartilage, and ligaments.
Safety and toxicology assessments are a critical component of efficacy trials in preclinical studies. Substantial evidence suggests that the anatomical, physiological, and biochemical function of pigs are similar enough to humans for regulatory authorities to accept the pig as an alternative nonrodent species for use in pharmaceutical safety and toxicology studies when scientifically appropriate. The TNRR Laboratory has experience in performing a wide range of assessments in Yucatan biomedical and commercial swine breeds. We also have access to a full-service laboratory to enable each client to have all aspects of a study performed by a single research entity.

Please do not hesitate to contact us with your inquiries so we may discuss all available options.