Every year, approximately 795,000 people suffer from a stroke and more than 140,000 people die, thus making stroke the fifth leading cause of death in the United States1. Although hundreds of therapeutics have sought to mitigate patient morbidity and mortality, few Food and Drug Administration (FDA)-approved therapies are currently available to treat ischemic stroke patients. The limitations of these therapies have promoted the continued investigation of novel ischemic stroke therapies.
In order to improve preclinical translation, the Stroke Therapy Academic Industry Roundtable (STAIR) and the Stem Cell Emerging Paradigm in Stroke (STEPS) consortiums strongly recommend testing in gyrencephalic, large animal models 2,3. Consequently, the TNRR Laboratory has developed a pig ischemic stroke model with brain anatomy and pathophysiology similar to humans4. Our research has provided evidence that pigs and humans share the following stroke pathophysiologies:
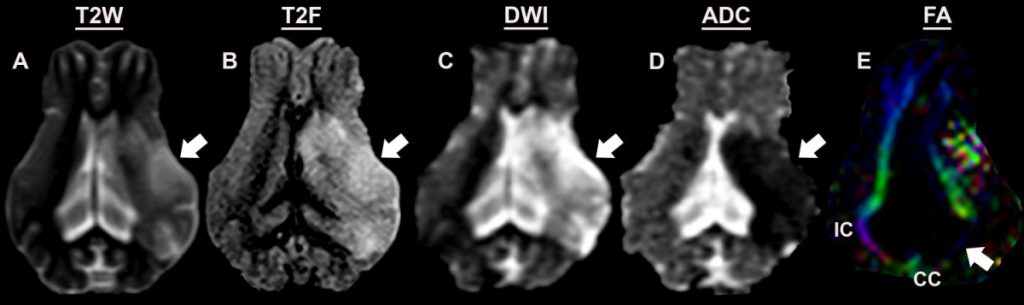
- Edema formation, hemispheric swelling, and midline shift as determined by T2Weighted (T2W, A) and T2Fluid Attenuated Inversion Recovery (T2F, B) images 7
- Lesion volumes as assessed via acute diffusion weighted imaging (DWI, C)6
- Primary onset of cytotoxic edema followed by delayed vasogenic edema as assessed via apparent diffusion coefficient (ADC, D)5
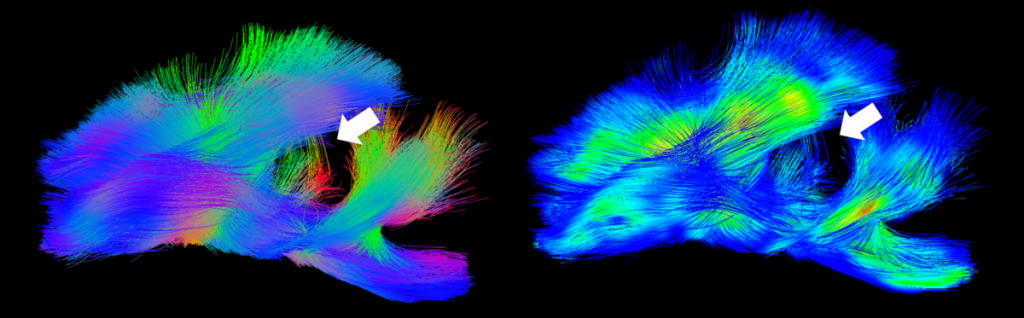
- Reductions in white matter integrity at acute and chronic time points as assessed by fractional anisotropy (FA, E) 7,9
- Cerebellar herniation8
- Deteriorations in spatiotemporal and relative gait analyses including velocity, cadence, swing percent of cycle, stride length, cycle time, and mean pressure7,10
Preservation of these pathologies is critical as they are frequently associated with poor neurological outcome, functional deficits, and premature mortality in patients11-14. Understanding how ischemia leads to these tissue-level changes and consequent cognitive and motor function deficits, preferably in models with comparable cerebral anatomy, is a research priority that will help advance strategies and preclinical testing of novel therapeutics for tissue repair and regeneration post-stroke. Click here to watch a video on how the TNRR Laboratory is researching and treating ischemic stroke with regenerative cell therapies.
- 1Virani, S.S., et al., Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation, 2020. 141(9): p. e139-e596.
- 2Savitz, S.I., et al., Stem Cell Therapy as an Emerging Paradigm for Stroke (STEPS) II. Stroke, 2011. 42(3): p. 825-9.
- 3Fisher, M. and R. Stroke Therapy Academic Industry, Recommendations for advancing development of acute stroke therapies: Stroke Therapy Academic Industry Roundtable 3. Stroke, 2003. 34(6): p. 1539-46.
- 4Platt, S.R., et al., Development and characterization of a Yucatan miniature biomedical pig permanent middle cerebral artery occlusion stroke model. Exp Transl Stroke Med, 2014. 6(1): p. 5.
- 5Kaiser, E.E., et al., Characterization of tissue and functional deficits in a clinically translational pig model of acute ischemic stroke. Brain Res, 2020. 1736: p. 146778.
- 6Kaiser E.E., W.F.D., Large animal ischemic stroke models: replicating human stroke pathophysiology. Neural Regen Res, 2019.
- 7Webb, R.L., et al., Human Neural Stem Cell Extracellular Vesicles Improve Recovery in a Porcine Model of Ischemic Stroke. Stroke, 2018. 49(5): p. 1248-1256.
- 8Spellicy S., K.E., Bowler M., Jurgielewicz B., Webb R., West F., Stice S., Neural stem cell extracellular vesicles disrupt midline shift predictive outcomes in porcine ischemic stroke model.
- 9Baker, E.W., et al., Induced Pluripotent Stem Cell-Derived Neural Stem Cell Therapy Enhances Recovery in an Ischemic Stroke Pig Model. Sci Rep, 2017. 7(1): p. 10075.
- 10Duberstein, K.J., et al., Gait analysis in a pre- and post-ischemic stroke biomedical pig model. Physiol Behav, 2014. 125: p. 8-16.
- 11Sanak, D., et al., Impact of diffusion-weighted MRI-measured initial cerebral infarction volume on clinical outcome in acute stroke patients with middle cerebral artery occlusion treated by thrombolysis. Neuroradiology, 2006. 48(9): p. 632-9.
- 12Gonzalez, R.G., Clinical MRI of acute ischemic stroke. J Magn Reson Imaging, 2012. 36(2): p. 259-71.
- 13Ahmad, A.S., et al., Considerations for the Optimization of Induced White Matter Injury Preclinical Models. Front Neurol, 2015. 6: p. 172.
- 14Lee, K.B., et al., Brain lesions affecting gait recovery in stroke patients. Brain Behav, 2017. 7(11): p. e00868.