Although alterations in gut microbiota are commonly associated with neurological diseases, changes in the microbial community post-neural injury have not been well characterized. As such, the TNRR Laboratory has investigated the changes in the gut microbiota composition and diversity using both our ischemic stroke and traumatic brain injury (TBI) pig models.
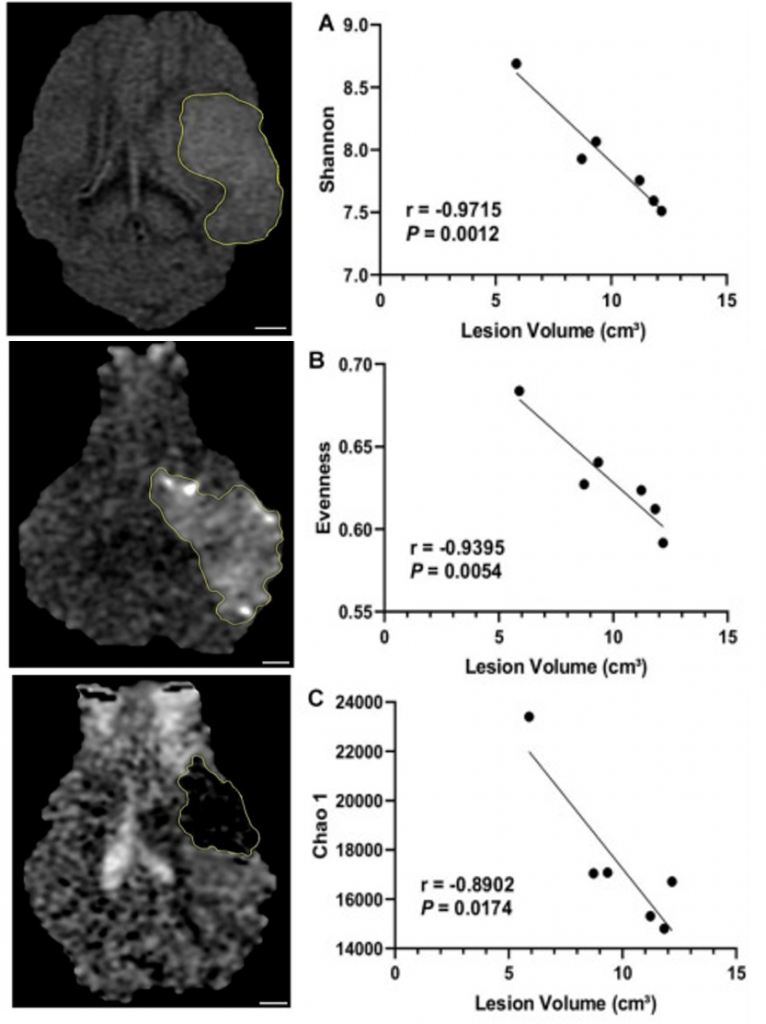
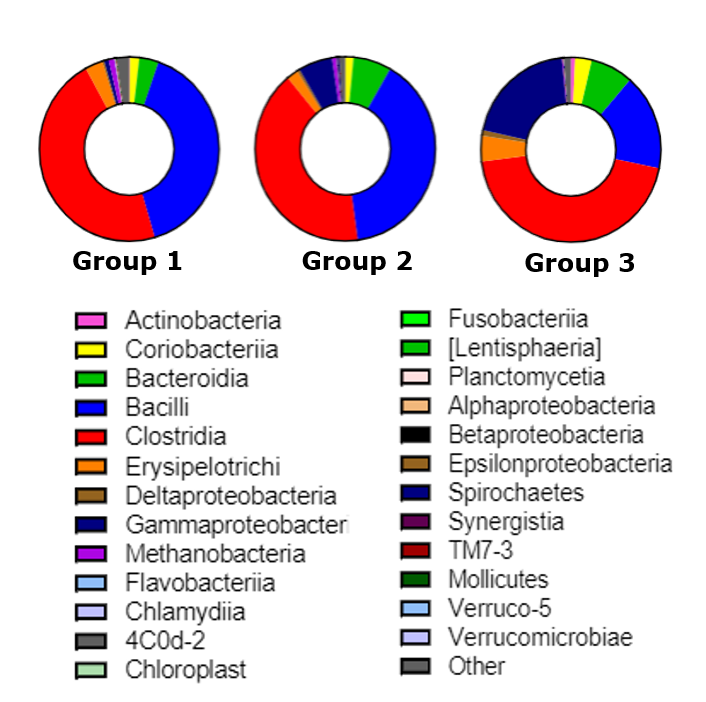
Post-stroke microbiota changes have been assessed in collaboration with the Bioactive Food Compounds and Health Laboratory. Specifically, the TNRR Laboratory determined that microbial diversity and evenness were reduced as early as 1 day post-stroke, while 3 days post-stroke microbial diversity negatively correlated with lesion volume1. Moreover, beta-diversity analysis revealed trending longitudinal differences, with the most significant changes in microbial patterns observed between pre-stroke and 3 days post-stroke. Abundance of the Proteobacteria was significantly increased, while Firmicutes decreased at 3 days post-stroke, compared to pre-stroke populations. Abundance of the lactic acid bacteria Lactobacillus was reduced at 3 days post-stroke. These adjustments in the gut microbiome have been found to influence ischemic brain injury by altering immune homeostasis and neuroprotective cytokine production2,3.
By day 5 post-stroke, the microbial pattern returned to similar values as pre-stroke, thus suggesting the plasticity of gut microbiome in the subacute stage post-stroke. Collectively, these findings provide a basis for characterizing gut microbial changes post-stroke, which can be used to discern stroke pathologies as well as provide potential therapeutic targets.
TBI-induced microbiota changes have been assessed via manipulation of the microbiota-gut-brain axis (MGBA). Previous studies have manipulated the MGBA by introducing specific probiotic bacteria which proved to be an effective TBI treatment in rodents. Specifically, treatment of TBI rodents with Clostridium butyricum and Lactobacillusacidophilus led to the establishment of microbiome profiles that correlated with decreased TBI lesion volumes, blood brain barrier permeability, and improved functional recovery4-6. To further explore these potential therapeutic targets, the TNRR Laboratory is seeking to replicate these findings in our pig TBI model.
- 1Jeon, J., et. al., Dynamic Changes in the Gut Microbiome at the Acute Stage of Ischemic Stroke in a Pig Model. Frontiers in neuroscience, 2020. 14: p. 587986.
- 2Benakis C., et. al., Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat. Med. 2016. 22: p. 516–523.
- 3Singh V., et. al., Microbiota dysbiosis controls the neuroinflammatory response after stroke. J. Neurosci, 2016. 36: p. 7428–7440.
- 4Nicholson, S.E., et al., Moderate Traumatic Brain Injury Alters the Gastrointestinal Microbiome in a Time-Dependent Manner. Shock, 2019. 52(2): p. 240-248.
- 5Ma, Y., et al., Lactobacillus acidophilus Exerts Neuroprotective Effects in Mice with Traumatic Brain Injury. J Nutr, 2019. 149(9): p. 1543-1552.
- 6Li, H., et al., Clostridium butyricum exerts a neuroprotective effect in a mouse model of traumatic brain injury via the gut-brain axis. Neurogastroenterol Motil, 2018. 30(5): p. e13260.