Inactivation of Human Coronaviruses
While COVID-19 unfolds, the food industry across the production chain is facing unprecedented challenges, ranging from infections in workers to many unknowns related to the virus inactivation, transmission, and potential impact on food safety. For this purpose, our goal is to investigate (1) the inactivation rate of infectious SARS-CoV-2 in relevant environmental matrices, (2) on surfaces commonly found in food production/services environment and on food under relevant environmental/production conditions, (3) the efficacy of commonly used sanitizers/disinfectants, and (4) the potential for several animal and common cold coronaviruses to serve as surrogates for SARS-CoV-2. The outcome of this research will be tailored interventions to inactivate the virus, which will benefit the food industry, public health and the national economy.
News article covering our Human Coronavirus research
Foodborne viruses

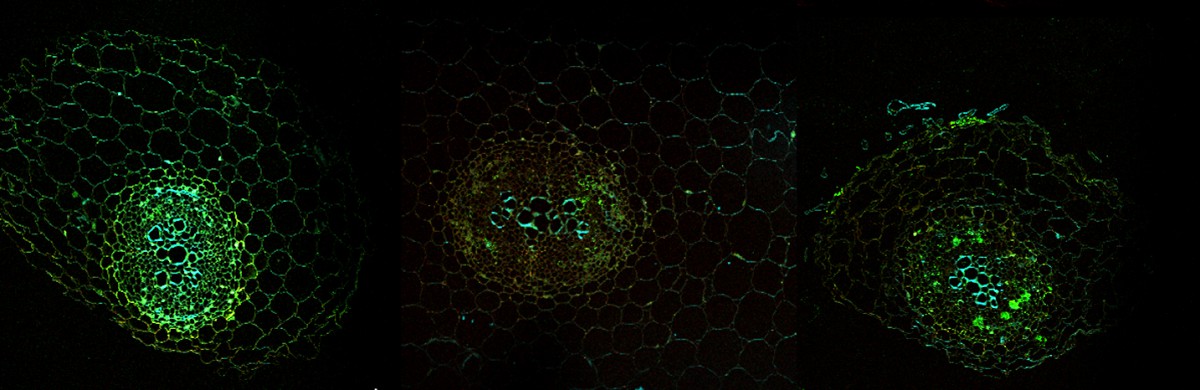
The World Health Organization estimates that 37,000 deaths occur annually due to foodborne viruses, especially human noroviruses. The Center for Disease Control and Prevention estimates that the majority of the foodborne illnesses in the USA are caused by human noroviruses, with an estimated 15,000 hospitalizations and 150 deaths annually and over $2 billion in health-care costs. Human noroviruses are often featured in the news as “stomach flu” or “Winter stomach bug”. These viruses can be transmitted via water, food, fomites, and vomitus and from person to person. Because of low infectious dose, a few viral particles can cause sickness with gastrointestinal illness (diarrhea, vomiting, stomach pain and nausea). In the USA, most of human noroviruses foodborne outbreaks that can be attributed to single food commodities are associated with fruits and vegetables (specifically lettuce and other leafy greens). Research conducted by Dr. Esseili revealed that HuNoV viral particles can persist on leafy greens (lettuce and spinach) for weeks and a number of factors can enhance the virus persistence on the surfaces of leaves, including the presence of phytopathogens and physical damage. The virus was also shown to internalize through the roots of lettuce and spinach seedlings into the leaves. In addition, virus-like particles of human norovirus were shown to bind specifically to lettuce cell wall carbohydrates extracted from leaves. Interestingly, those carbohydrates mimic human histo-blood group antigen (HBGA) carbohydrates which are risk factors for human infections. The Esseili lab is conducting fundamental research to answer questions regarding virus transmission in the environment (from farm to fork) and viral interactions with leafy greens and other susceptible fruits (such berries) in order to come up with innovative solutions to disrupt the virus transmission through food and water.
Environmental transmission of human pathogens

Contamination of water resources with human pathogens represents one of the most significant global public health challenges. In developing countries, problems associated with contaminated water are alarming, as billions of people either lack access to safe domestic water or have little or no means for sanitation. Impaired waters result in millions of deaths annually (mostly children and immuno-compromised people), related to diseases associated with human and animal waste. Dr. Esseili has optimized a microbial community fingerprinting technique for tracking the transmission of the fecal pollution indicator, Escherichia coli, in surface waters. The optimized method generates a “fingerprint” descriptive of various E.coli populations in different geographical locations (ditches, creeks, storm drains, etc.) of a given watershed as well as populations in wildlife and farm animals and human sources (such as wastewater treatment plants effluents or biosolids). The method allows analysis of temporal dynamics of persistent E.coli populations and exposes shifts in the population in response to environmental stressors or new/transient or persistent sources of fecal pollution. Using this method, Dr. Esseili’s research provided a better understanding of the transmission routes of fecal pathogen indicators within a number of watersheds. Additionally, the FSMA Produce Safety rule focuses on the prevention of foodborne pathogens and requires establishing a microbial profile (using the fecal pollution indicator, E.coli) to assess agricultural waters used for growing produce or for direct/ indirect contact with produce. To this end, the Esseili lab continues to explore ways to understand the transmission of fecal indicators and viral surrogates in relation to actual human pathogens for the purpose of enhancing the safety of agricultural waters and reducing the burden of waterborne and foodborne illnesses.